Cadmium, copper, lead, mercury, nickel, silver, zinc: all are metals that have a place in our industrialized world. But they also can be toxic to the living things of our air, land and sea…particularly when they occur in concentrations typical of urban environments like New York City.
As far back as the 1880s, tons of mercury from industrial processes were dumped into parts of the New York / New Jersey Harbor Estuary (the Hudson-Raritan basin) annually, culminating in the high levels of contamination associated with the mid-1970s. But due to a greater awareness of these contamination issues, the implementation of clean air and water legislation, and more stringent pollution prevention initiatives, mercury discharges are now estimated to be less than those that occurred in the 1880s. However, high concentrations of some contaminants still remain in some sediments [Hudson River Foundation’s report, Health of the Harbor: The First Comprehensive Look at the State of the NY/NJ Harbor Estuary 2004].
Today, municipalities and communities are trying to restore bays and marshes to provide better habitat for native species. However, it appears that the food webs taking shape are not as they may have been in a pre-industrial time. Species that are sensitive to metals may have already disappeared from the scene, while those that are tolerant of such conditions can often dominate these impacted systems. A research team from the College of Staten Island led by Dr. William G. Wallace and Sea Grant Scholar Daisuke Goto looked at how metals move up the trophic levels of a food chain. In a series of research papers published over the last several years, this team observed that local invertebrates have different ways of storing metal internally and that this internal storage and the digestive processes of their predators can control the transfer of metal up food chains.
Wallace and his students took samples near the College of Staten Island and its environs, including the industrial Arthur Kill. (Note: “Kill” is Dutch for “stream” and was so named when New York was New Amsterdam.) They collected common species like shrimp and worms and found that generally these invertebrates tolerate metals. “They can handle some level of contamination because they internally detoxify the accumulated metal by binding it to a special protein or by producing ‘kidney stone’ type concretions,” says Wallace.
Although metal concentrations in the water column have decreased significantly over the last three decades, local water bodies such as the Arthur Kill still have elevated levels. For instance, according to the Health of the Harbor report, although mercury loadings into New York Harbor have decreased in the last few decades, sources of this toxic metal still exist from wastewater, landfills, and airborne release from power plants. Furthermore, mercury from previous contamination events may remain in the sediments and become biologically available, either as inorganic mercury or the more dangerous methyl mercury.
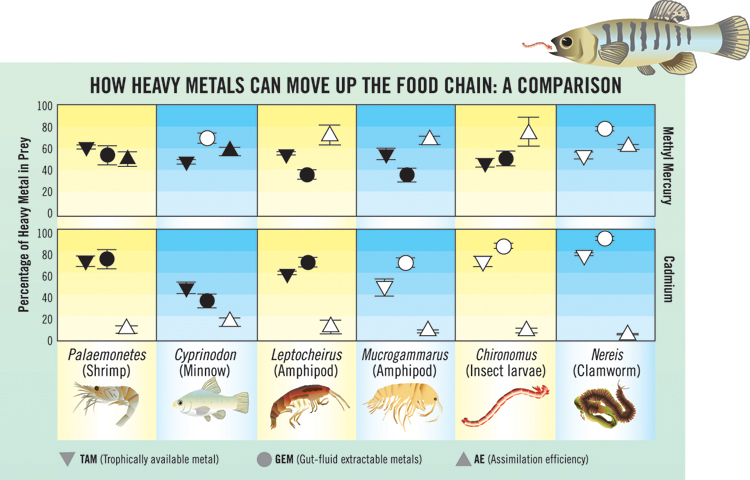
The chart compares how two kinds of metal (methyl mercury and cadmium) are transferred to mummichogs after they consumed a variety of prey. Metal transfer occurs in three steps: 1. Metal is bound to the TAM of prey (percentage shown by an upside down triangle). 2. Metal is released into the gut fluid of the predator (circle) and is free to be assimilated. 3. Then, if the conditions are right for transfer across the gut lining, the metal is indeed assimilated by the predator (triangle). When a fish consumes a shrimp, the percentages of mercury available in the tissue, released into gut fluid, and assimilated by the predator are similar in value; these similarities are indicated by the darkened symbols. The results are quite different for cadmium; although there is available metal in the tissue and in the gut fluid, very little metal is assimilated.
According to Wallace, methyl mercury is a driving force in creating the food web found in the moderately polluted creeks surrounding the Arthur Kill. A common small predator is the mummichog, a chubby little fish that that travels in schools in marshes along the northeast coast. (The name comes from a Native American phrase meaning “going in crowds.” People use them for bait, too, and usually refer to them as killifish or “killies.”)
Because mummichogs lay their eggs near the high tide mark where they are exposed to the air, their tolerance for poor, low-oxygen conditions make them an important part of the food web in the polluted marsh ecosystems, preying on small invertebrates and minnows and then in turn, becoming prey to larger fish or shore birds on the next trophic level of a food chain. Wallace and his team used these fish in a series of experiments to examine the interactions between mummichogs and their prey. Some of their results are summarized on the chart.
Let’s say a mummichog swallows a worm and begins digesting it. Enzymes break down the tissues and proteins, releasing the metal. In the case of the worm, a very large percentage of the metal within its tissues is bound to proteins making the metal readily available to predators. Scientists call this the trophically available metal fraction or TAM. Once digestion has taken place, breakdown products become soluble and are free to move across the gut lining and become assimilated into the fish. In the case of methyl mercury, almost all of the available metal (TAM) comes out of the protein and crosses the gut lining. That happens because the mercury is bound to an organic portion which makes it fat soluble and thus compatible with the lipid layer of the gut lining.
In the case of cadmium, while the amount of the metal bound as TAM in prey and the amount released into gut fluid (for some prey) can be nearly equal, very little of this metal is taken up by the fish. “That’s because cadmium cannot so easily cross the gut lining, which tempers the assimilation of the metal,” says Wallace.
There are several different factors that come into play in determining how metals are transferred from one trophic level to the next. First there is the metal in the sediment or the water column and how much of that the prey takes in. Then the prey may have some way of detoxifying the metal, like putting metal into granules or binding it to proteins. These are both prey-dependent and prey-specific factors. Then there are factors that are predator-dependent like the predator’s physiology, digestion process, and assimilation rates.
Knowing which organisms are able to sequester and detoxify metal contaminants may be of interest to those municipalities doing habitat restoration work in urban areas. And knowing which predators digest and assimilate metals from their prey and those that excrete the metals back into the water column might also be of interest to managers planning restoration efforts. Although metal concentrations in the water column have been going down, sportfish consumption advisories for fish caught by anglers in contaminated areas like the Arthur Kill have been in effect for decades and will likely continue for some time to come.
— Barbara A. Branca